What is smalt?
Smalt was an important pigment in European oil painting, particularly in the sixteenth and seventeenth centuries.1 Its origins probably lie in the blue pigment used by the ancient Egyptians, known as us as ‘Egyptian blue’; both pigments are made from glass that has been coloured blue and both are also used as glazes on ceramics. However, Egyptian blue contains copper, whereas smalt derives its colour from cobalt.

Fig 1. Ceramic cobalt glaze
Being a glass, smalt is transparent and the colour is never strong, although this varies depending on how much cobalt is present. The pigment comprises large particles and is quite coarse. If ground too fine the colour would be weakened, and both artists and their colourmen would have known that it should be ground carefully by hand, and not for too long. It provided a much cheaper alternative to the expensive mineral pigment ultramarine
The largest particle ever found at by a Tate conservator was on a painting by Turner. It measured an impressive 70 micrometres across. Normally, ‘large’ particles measure 10-20 micrometres across. A 70-micrometre particle tends to stick out of the paint film, conferring texture on the surface, and can be seen even without a microscope.
The scientific properties of smalt
Smalt is manufactured by thoroughly mixing cobalt oxide with molten glass. Potassium, in the form of potassium carbonate is added to the glass to act as a flux – it makes it flow more easily. Once thoroughly combined, the hot glass is added to water. The temperature shock causes it to shatter into small pieces. The pieces are then ground to yield a coarse pigment. Smalt is an ideal pigment for glazing ceramics as it is nearly transparent (fig.1).
An inorganic pigment that does not have a precise chemical formula, smalt contains between 2 and 18% cobalt oxide and 66 and 72% silica. The potassium content varies from 10 to 21%.2 Impurities can include oxides of copper, magnesium, sodium, nickel, manganese and barium.3
Research into why smalt fades
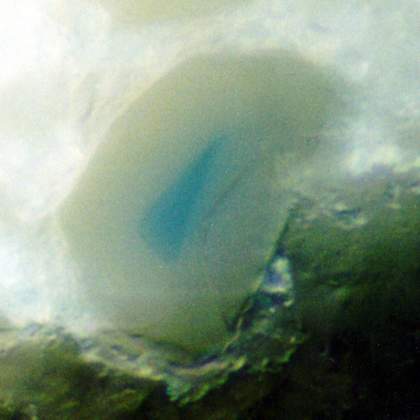
Fig 2. Particle of discoloured smalt with an un-faded core seen in cross-section
Although smalt is known to fade, the degree to which this occurs can vary even within the same painting. Research carried out by Professor Jaap J. Boon, honorary scientific advisor to Tate, and his collaborators, has started to reveal why fading in the pigment occurs.4
A clue is provided by the observation that particles have been found where only the core of the pigment retains the blue hue (fig. 2). Scanning electron microscopy revealed that despite the lack of colour on the outer part of the particles, cobalt is in fact still present and evenly distributed across the particle. It had been assumed by some that fading resulted from leaching out of the cobalt content.
However, it can be seen that it is the concentration of potassium (K) that is crucial (fig. 3). Where the ratio of potassium to cobalt (Co) is 1:1 or higher, the colour has not faded. The data for this research was obtained from cross-sections taken from five sixteenth-century paintings.
The impact of a high proportion of potassium in smalt
Potassium is strongly alkaline and these findings suggest that a certain level of alkalinity is required to maintain the blue colour. Pigments are suspended in oil which is a slightly acidic environment. A small proportion of fatty acids are present in the oil and it is probable that these acids react with the potassium.
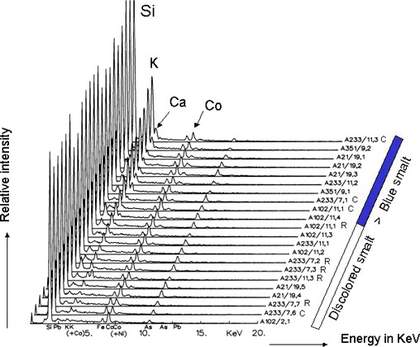
Fig 3. SEM-EDX data of smalt in paint cross-section ordered according to increasing relative amount of potassium.
The colour transition from blue to discoloured corresponds to a potassium: cobalt ratio of approximately 1:1.
Reproduced by courtesy of J.J. Boon et al.
Moisture must be present for this reaction to take place, but potassium is known to attract moisture and there will be occasions when the amount of moisture in the air is likely to be high. The potassium in the centre of the particle will react last, enabling the pigment to retain its colour for longer.

