A picture lives by companionship, expanding and quickening in the eyes of the sensitive observer. It dies by the same token. It is therefore a risky act to send it out into the world. How often it must be impaired by the eyes of the unfeeling and the cruelty of the impotent who would extend their infliction universally.
Mark Rothko, 19471
On the 7 October 2012 Mark Rothko’s Black on Maroon 1958 (Tate T01170), one of the Seagram Murals, was tagged with a highly fluid, heavily coloured black ink (figs.1 and 2). Tate’s nine Seagram Murals have iconic status within the collection and although only one of them was targeted, their interrelatedness meant that the vandalism was particularly devastating. The visible damage was significant and as the painting was delicate, degraded and unprotected by glazing or a coherent varnish layer, the attack presented a serious conservation challenge. In the days following the incident, much discussion ensued as to the most appropriate method for reversing the damage. The treatment team began with a review of existing research on Rothko’s paintings, which was then combined with new investigations into the effect of the graffiti on the painting’s surface. Little was known about the ink, its properties and most importantly its reversibility, and so research into the characteristics and solubility of the ink was also undertaken. The testing and application of potential solvent systems could not be carried out on such a delicate painting, hence it was decided that a testing surface – a ‘representative sample’ – would have to be devised. The test sample had to behave in a manner similar to the painting and so needed to comprise a similar stratigraphy and materials. To achieve this, an in-depth study of Rothko’s working practice, his materials and techniques was carried out.

Fig.1
Mark Rothko
Black on Maroon 1958 after vandalism of 7 October 2012
Tate
© Kate Rothko Prizel and Christopher Rothko, 2012

Fig.2
Detail of graffiti in bottom right of Black on Maroon 1958
Tate
© Kate Rothko Prizel and Christopher Rothko, 2012
Rothko’s working methods
Little is known of Rothko’s technique and he preferred it that way. He exalted in the idea of secrecy and silence. As he himself put it, ‘some artists want to tell all like at a confessional. I as a craftsman prefer to tell little’.2 Elizabeth Jones, a conservator who helped Rothko install his Harvard Murals in 1964, remembered that attempts to ask him about his technique or materials were swiftly dismissed as too ‘professional’ an enquiry.3
Rothko was concerned with the permanence of his paintings. Roy Edwards, his assistant when working on the Harvard Murals, remarked in 1971 that ‘He was always reading these books on how to make paintings more permanent. Very concerned with that’.4 However, as has also been well documented, Rothko was experimental and innovative with his materials, making choices that were on occasion at odds with his concerns for longevity.5 He chose light fugitive materials, either consciously or by necessity, if they suited his desired aesthetic effect.6 Although clear evidence of the artist’s hand in the making of a painting was essential to Rothko, he sought to enigmatise his processes. Most likely, he wished for the viewer to be unencumbered with contemplating all too obvious remnants of his technique, but rather to be transported beyond their physical making.7
Technical research can be viewed as an exposure of an artist’s materials and practice, sometimes against their express will. Hence the value of revealing a once guarded process should be carefully weighed against the benefits it can bring to any necessary conservation treatment and subsequent dissemination of this information.
Mock-ups and reproductions as tools for evaluating conservation treatments
Mock-ups and reproductions are occasionally used as conservation tools to understand the structure of complex artworks, the materials involved in their construction, and the techniques employed by the artist. They can also provide useful test surfaces for conservation treatments. Modern and contemporary paintings can have vulnerable surfaces that do not lend themselves to discrete testing. These ‘ersatz’ tools allow for testing comprehensive and sometimes unorthodox processes on what are often equally unorthodox original materials, thereby limiting the probability of damaging the original object. Good examples of cases in which ‘ersatz’ tools have facilitated successful treatments and have added to scholarship on an artist’s techniques are Christa Haiml’s investigation of reversible methods for the re-integration of losses to the matt and textured surface of Yves Klein’s Blue Monochrome in the Menil Collection, Houston, and Glenn Alan Gates’s reproductions of paintings by Morris Louis for the evaluation of stain removal techniques.8 Therefore a key initiative in the treatment plan was to create a sample using materials and techniques representative of Rothko’s painting in order to better understand the original and to provide an appropriate surface for testing ink removal systems.
Making the representative sample

Fig.3
Schematised design for the representative sample
© Tate
The primary aim of the conservation treatment was to remove black ink graffiti from the bottom right corner of the canvas while minimising as much risk to the painting as possible. The treatment team initially considered producing a mock-up that would ideally look, as well as behave like the painting. However, it was quickly established that it was not possible to mimic Rothko’s idiomatic practice and technical prowess. In addition, we could not source historically accurate, original materials. Instead we decided to make a sample that ‘represented’ the knowledge available of the materials and techniques of Black on Maroon (fig.3). In particular, we relied on technical and scientific research that had been carried out in preparation for the 2008 Rothko exhibition at Tate Modern.9 The 2008 campaign was the culmination of previous attempts to examine and analyse the Seagram Murals and resulted in a useful understanding of the layer structure of the paintings and the identification of the majority of the materials Rothko used, including animal glue, egg, dammar, oil-modified alkyd resin and possibly modified phenol formaldehyde resin. However, these analyses ultimately yielded uncertain results, due to the fact that Rothko painted in very thin layers using a variety of paint media. The analysis of multifarious materials can prove challenging as it is difficult to isolate particular materials within the range of results. Nonetheless, the 2008 exhibition catalogue contains a table summarising the analytical results into the materials present in each of the Seagram Murals, including a list of pigments and media found in Black on Maroon.10 However, the exact layering of these materials, the comparative thickness of the graffiti ink and whether or not the degree to which it had penetrated the paint layers also needed to be established.
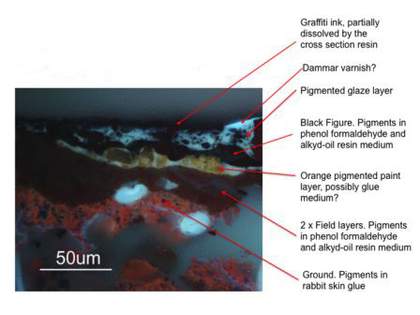
Fig.4
Cross-section x500 UV from graffiti-covered black ‘figure’ paint of Black on Maroon 1958 with annotated layer sequence (based on information published in the 2008 exhibition catalogue)
Image: Jaap Boon
© Tate
Previous minute paint cross-sections taken from the painting had yielded confusing results partly due to the extremely friable nature of the paint layers, but also because of the tendency for samples to fragment upon extraction. Further difficulties arose from the fact that the paint layers were applied very thinly and using a wet on wet technique. It was therefore decided to take a slightly larger cross-section sample, which did not fragment and allowed a better understanding of the ink-affected area (fig.4). This new section revealed more accurate information about the layer structure, enabling us to proceed with the fabrication of a test painting with a more reliable stratigraphic as well as material approximation of the original work. Based on all previous analyses and new technical, scientific and visual information, a design for the representative sample was schematised as shown in fig.3. On the right-hand side, the first layer is shown partially exposed, followed by a sequenced system of layers representing those of Black on Maroon, building up from the pigmented size layer (right) to the final resin glaze (left).
Following Rothko’s preferred choice of painting support, a heavy-weight cotton duck ‘awning-type’ canvas was acquired and attached to a wooden strainer (there seemed to be no benefit in replicating and applying Rothko’s ‘sprung’ wooden stretcher as found on Black on Maroon).11 Taking care to match the colour and consistency of the ‘ground layer’ of the painting, a size mixture consisting of rabbit skin glue, synthetic ultramarine and lithol red was prepared. This was heated to a consistency described by Rothko’s assistant Dan Rice: ‘like water this stuff’.12 The ground was brushed on with large decorators’ brushes, in imitation of Rothko’s technique.13 This layer saturated the cotton canvas as a translucent stain, satisfactorily matching that on the painting, albeit in a much more intense hue. The original lithol red pigment has undergone photochemical ageing and possibly deterioration caused by interactions between the lithol red pigment and other materials within the paint system.14 The maroon ‘field’ was the next layer to be added. Analysis and visual observation suggested that this layer comprised two separate applications of the same paint (see fig.4). The components of the field had been identified in 2008: the medium containing possible phenol formaldehyde and oil-modified alkyd resins, and the pigments including a combination of French ultramarine, barytes, kaolin, iron oxide, cadmium red, bone black and lithol red.15
While oil-based alkyd decorative paints were commonly used in the 1950s, phenol formaldehyde is a more unusual binder to find in fine art painting. Phenolic-based decorative paints were available from the early 1930s and by the 1950s they were being used as a medium for off-set printing inks and in ‘quick-dry’ gloss and flat decorative paints.16 Elizabeth Jones recalled that ‘when he [Rothko] ran out of paint he went downstairs to Woolworth’s and bought more … he didn’t know what kind it was’.17 It is possible that Rothko would have been attracted to a commercial product claiming to be a rapid drying paint as this would have facilitated the fast build-up of paint layers. Perhaps it permitted him a more expressive and gestural approach than most other available media. However, we cannot be sure how Rothko came to add this material into his painting (further technical investigations of the media of Black on Maroon are underway), which is one of the reasons why we could not produce an ‘historically accurate’ reproduction of the painting.
In an effort to recreate paint with as similar materials and properties to the original as possible, a supplier of modified phenol formaldehyde resin was located. This resin is used in printing applications and dissolves slowly in Shellsol T (Shellsol T being similar to artists’ turpentine – the most likely diluent used by Rothko). The oil-modified alkyd medium was purchased from Kremer Pigmente in Germany. According to the 2008 analysis phenol formaldehyde was found to be present in ‘abundant quantity’, and so the ratio of media chosen was two parts modified phenol formaldehyde in Shellsol T to one part oil-modified alkyd (used as supplied). In combination they produced a viscous, yellow medium and direct experience of dissolving these materials suggests that it is very unlikely that Rothko made up this paint medium himself. The subsequently admixed pigments were balanced to colour-match the ‘field’ colour of the painting and further dilution with Shellsol T was made where necessary. One application was brushed on top of the ground layer of the sample, again with five-inch decorators’ brushes. After drying for twenty-four hours, a second layer was added. An attempt was made to mimic Rothko’s technique when applying the ‘field’ layers, following Dan Rice’s recollection that ‘the physical movement was very active and very graphic’.18 The resulting ‘field’ paint film of the representative sample was glossier and a deeper red colour than that of the painting, but had a consistency and layer thickness close to the original.
In previous visual examinations a dammar glaze had been identified between the maroon field and a thin layer of orange paint, which may have been applied to seal the underlying ‘field’ paint. Consequently a film of dammar resin was next applied to the representative sample. In addition, a heretofore undocumented orange paint layer was also noted at the feathered edges of the black form; although this had not been seen on previous cross-sections of the painting, it was visually quite evident. At the time of construction, however, neither the orange pigment nor its medium had been identified. Therefore this layer was simulated using cadmium orange pigment in the same modified phenol formaldehyde/alkyd oil medium.
The black paint of the ‘figure’ was the final paint layer to be applied, again a mixture of phenol formaldehyde/oil-modified alkyd medium and kaolin, barytes, bone black as well as iron oxide and earth pigments. This combination of pigments was added to the medium and both body and colour were once again matched to the ‘figure’ paint on Black on Maroon.
Finally, the two uppermost glazes, the first consisting of whole egg and the second of dammar, were applied. The order of application of these glazes was based on careful visual examination of the painting, particularly in ultraviolet light. It appears that in most areas the dammar glaze extends over the egg glaze, as revealed by the differently fluorescing colours present in fig.4.

Fig.5
Representative sample after completion
© Tate
After completion, the sample was cut into sections to fit into Tate’s accelerated ageing chambers. Two samples containing all of the applied layers and two samples containing mostly the field and ground layers were installed. Each sample was subjected to a combination of light ageing for the equivalent of fifty years in museum display conditions, with added thermal ageing at 30°C and relative humidity cycling from 55–85% over a twenty-four hour period. The aged samples were then tagged with the same graffiti ink used by the vandal. At this stage the preparation of the test samples was completed (fig.5). A cross-section of the aged sample, containing all layers of paint, which had then been tagged (fig.6), can be compared with the cross-section from the painting (fig.4). Note the similar micro-cracks in the graffiti ink and underlying paint film.
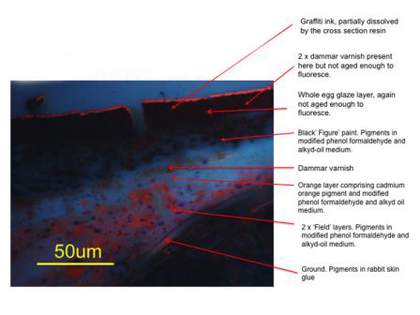
Fig.6
Cross-section x500 UV from artificially aged representative sample containing all layers and graffiti
Image: Jaap Boon
© Tate
The constituents and solubility of the ink
Designing an appropriate ink removal strategy necessarily began with an investigation of the ink. The product used to deface the painting was Molotow Coversall Cocktail ink, described by the manufacturer as visco-plastic, alcohol-based, 100% buff-resistant, ultraviolet light resistant, quick drying, permanent and weatherproof.19 The colour is listed as ‘signal black’ and the colourant is described as ‘nitrogen synthetic bitumen’ and was applied using a marker that facilitated rapid application, producing vertical drips in addition to the inscribed letters (see fig.2).
Analysis of this unusual, proprietary ink material is ongoing. However, it appears to be based on an aromatic hydrocarbon resin, with a high proportion of black dye colourant and polar solvents identified as a 2:1 mixture of ethanol and 1-methoxy-2-propanol (propylene glycol mono methyl ether) with other solvents (e.g. methanol, 2-butanone and ethyl acetate) present in trace (>1%) amounts.20
The areas covered by the graffiti ink included the mostly unglazed, porous and heavily oxidised maroon paint (containing cadmium red, lithol red [PR49] and ultramarine blue) as well as the oxidised egg and dammar glazed black ‘figure’ paint (containing bone and Mars blacks and extenders). The valuable cross-section (fig.4) revealed that the ink had not uniformly penetrated through all paint layers. This was partially due to the fast drying rate of the ink in addition to Rothko’s application of dammar and egg glazes. Nonetheless, the presence of micro-cracks in the paint layers facilitated the flow of ink through to the cotton canvas support in several areas, and it was also evident that the dammar resin glaze at the uppermost surface had been dissolved by the ink solvents.
High magnification observations and scratch tests of dried ink samples revealed that the ink was prone to brittle fracture and therefore of low molecular weight. Simple paper chromatography experiments revealed that the ink remained highly soluble in methanol, ethanol, isopropanol and acetone after drying, with no evidence of separation. Encouragingly, the black colourant moved with the solvent front. However, minute agglomerates of black particles remained visible within paper fibres at low magnification.21
In order to determine whether the ink was likely to quickly become insoluble (hence making the need for removal urgent), a simple solubility test was carried out. For this, the ink was applied to a commercial acrylic primed canvas and light aged to the equivalent of twenty years in museum display conditions (UV free, 21°C, 55% relative humidity). Swab tests carried out on both the light-aged and unaged control samples using methanol and acetone revealed that the solubility of light aged ink remained similar to the unaged control. This simple test, although by no means exhaustive, indicated that the ink was not likely to cross-link significantly within the projected time-frame of the treatment and become more difficult to remove. Hence the treatment team could carry out extensive testing prior to embarking upon the removal of the ink from the painting surface without any significant risk of the ink changing significantly with respect to solubility.
Helpfully, through an ongoing collaboration between Tate and the Dow Chemical Company, the Hansen Solubility Parameters (HSP) of the ink were determined through observation of the dried ink in eighteen solvents of different polarity. The HSP values obtained were: Hansen Dispersion Parameter (17.94); Hansen Polar Parameter (7.91); and Hansen Hydrogen Bonding Parameter (12.01); measured in (J/cc)1/2, as listed in Table 1.
Initial solvent selection
CHEMCOMP™ Solvent Property Modeling Service was used to facilitate solvent selection. The ink HSP values were entered into Dow’s solvent database and solubility predictions in the form of relative energy density (RED) values were generated. From over 600 solvents, a group of sixteen with high ink solubility predictions (i.e. low RED values) across a range of solvent classes were chosen within health and safety considerations (Table 1). The initial effect of each solvent was assessed at Dow through removal tests on ink applied to acrylic primed canvas and dried for four and thirty days in ambient conditions. For this, a few drops of solvent were applied to the surface and covered for ten to twenty seconds or two minutes prior to cleaning using a ready-made cotton swab. The same group of solvents were also tested on a two-year old solvent borne green alkyd paint (high RED values), which represented one of the media types in Black on Maroon prior to the availability of the representative sample.
As listed in Table 1, the general classes of solvents that had a useful effect on the ink included alcohols, ethers, esters and ketones. Those with a more effective cleaning performance on the ink (rated ++ and above) included: methanol, benzyl alcohol, methyl CARBITOL™ Solvent (diethylene glycol monomethyl ether or 2-(2-methoxyethoxy) ethanol), ethyl lactate, acetone, DMSO and ethylene glycol diethyl ether. Further tests at Dow into the bleeding behaviour of each solvent and the addition of two water-in-oil microemulsions (WiO) led to the creation of a top-five list. This included: acetone, methanol, ethyl lactate, INVERT™ 2000 Microemulsion (limonene-containing WiO microemulsion) and a mineral spirits-based WiO microemulsion containing linear alkylbenzene sulfonate (LAS) surfactant in a formulation similar to those trialed on acrylic paints.22
Empirical testing and solvent refinement

Fig.7
Test sample showing ink applied to commercially acrylic primed cotton duck canvas with some cleaning tests
© Tate
To further explore the top-five Dow solvent options they were incorporated into a larger group eventually consisting of over seventy-five systems (Table 2) evaluated across a range of substrates. The first evaluation (Phase 1) involved the application of ten swab rolls of each system to ink applied onto a primed titanium white acrylic canvas (fig.7). Assessments included observations on speed of ink removal, solvent and ink bleeding, paint swelling, solvent bleed to the back of the canvas, paint surface change, ease of solvent use and the effects of prolonged solvent use. From this, each solvent was assigned a rating from one to five, where five was the most successful.

Fig.8
Accelerated aged representative sample tagged with ink
© Tate
A selection of fourteen systems rated three and above were then selected for further testing (Phase 2) on the acrylic primed sample where the cleaning process was taken to either fifty swab rolls or a natural stopping point (e.g. swelling was noted). This included: benzyl alcohol, toluene, ethyl lactate, INVERT™ 2000 Microemulsion, INVERT™ 5000 Microemulsion, 1-methoxy-2-propanol, diethylene glycol ethyl ether, methanol, ethanol, industrial methylated spirits, acetone, methyl lactate, propylene glycol propyl ether and ethylene glycol diethyl ether. Some systems were then ruled out due to health and safety considerations (toluene) and others rejected due to their tendency to make the ink spread quickly (e.g. methanol, ethanol). Six solvents (benzyl alcohol, ethyl lactate, INVERT™ 2000 Microemulsion, 1-methoxy-2-propanol, diethylene glycol ethyl ether and diethylene glycol monophenyl ether) were then taken forward for testing (Phase 3) on ink applied to the accelerated aged representative sample (fig.8). Observations included: speed of ink removal and controllability, pigment pick up, paint swelling/damage, solvent/ink bleeding, ink staining and paint surface change. This group was then slightly expanded to include the other systems listed in Table 3.
Benzyl alcohol was eventually favoured for a number of reasons including its high affinity for dissolving the ink (low RED, Table 1), the ability to swell and dissolve the ink in a controllable way (due to its relatively high boiling point), minimal pigment loss and minimal changes in surface gloss. However, due to its low vapour pressure (Table 3), it took time to evaporate and occasionally resulted in mild blanching. Attempts were made to increase the solvent evaporation rate by adding proportions of short-chain alcohols. However, this reduced the ink removal efficacy. The ink solvent blend was also assessed for removal and, as anticipated, it dissolved portions of the ink too quickly, thereby increasing the risk of residual ink staining.
Phase 3 also encompassed explorations into the solvent application and removal processes. This included: the use of silicone solvents such as octamethyl cyclotetrasiloxane (D4) as a non-polar masking layer applied before the more polar solvent, blending or incorporating solvents into gels (e.g. Velvesil™ Plus and other silicone polymer gels, Pemulen™ TR2, hydrolised polyvinyl alcohol-borax polymer, agarose and xantham gum); the use of brushes and other tools; masking the ink with polymeric materials to prevent ink bleed (cellulose polymers, cyclododecane); as well as solvent and/or gel application through different types, weights and thicknesses of tissue.
After extensive testing, gelled systems were excluded as they did not offer a high enough degree of control and required clearance in addition to blocking visual access to the paint surface during application. Many of the gels removed some ink and slowed down the transport of the solvents. However, using free solvents offered more control over the solvent contact area, greater visibility at the paint surface, and avoided the need for clearance. The masking options did not prove necessary, as ink spreading was not a particular problem as long as the solvent application process was well controlled. The use of D4 as a non-polar barrier proved beneficial in terms of slowing down polar solvent contact. However, due to the low surface tension of D4, there was a tendency for the dissolved ink to spread with the solvent onto unaffected areas.
Prior to testing the top-rated systems on the painting, a large maroon pigmented-glue size primed canvas dating from around the same year as Black on Maroon was donated from the collections of Kate Rothko Prizel and Christopher Rothko. With permission, graffiti ink was applied to one area and it became immediately apparent that the ink sank further into the paint surface than it did on Black on Maroon. The systems listed in Table 3 were also evaluated on this sample (Phase 4) through the application of swab rolls. Observations included how many rolls were applied before pigment was picked up, speed of ink removal, bleeding of the ink through to the reverse, paint surface after removal, and the extent of ink staining in the paint. In this case, the phenyl ether and INVERT™ 2000 Microemulsion proved less effective than other systems. Although ink removal was encouraging with the WiO benzyl alcohol microemulsion, the surfactant content (TRITON™ XL-80N) resulted in foaming during clearance and also made it difficult to judge the paint surface. Not surprisingly, this sample proved more difficult to clean than the well-bound aged representative sample, but it proved particularly useful in refining the ink removal process from the maroon sections of Black on Maroon.
Removing the ink from Black on Maroon

Fig 9a
Hirox digital image of cleaning test on the black paint using benzyl alcohol through silicone solvent D4
Image: Emilien Leonhardt
© Tate

Fig.9b
Hirox digital image of cleaning test on the maroon red paint using benzyl alcohol through silicone solvent D4
Image: Emilien Leonhardt
© Tate
Due to differences in paint porosity and the thin oxidised glazes present on the black paint, the interaction between the ink and the two paint types on Black on Maroon slightly differed. Initial ink removal tests involved the application of benzyl alcohol through D4 using swabbing action. Cleaning tests were evaluated using a high resolution digital microscope (figs.9a and 9b). The images clearly show that the surface recovery after ink removal from the black paint was encouraging. However, the maroon paint proved more vulnerable to both solvent and mechanical action.
Subsequent testing involved most of the solvents listed in Table 3. These systems were applied in small areas, approximately 5 mm^2, using a size-0 sable-haired brush while observing the process under illuminated magnification. The brush was dampened in solvent and applied in one sweep across the designated area. Where the paint was more textured the brush was lightly oscillated to encourage dissolution of the ink. The solvent was left to swell the ink for five seconds, then a small thin piece of highly absorbent synthetic-fibre tissue was applied directly to the area to encourage uptake. This process was carefully repeated until the ink residue was no longer visible and/or the ink was reduced as much as possible while minimising any damage to the paint film. It is important to note that the degraded dammar glaze had been partially dissolved during the (alcohol-based) ink application process, and that every effort was made to minimise further changes to the paint film during the removal process.

Fig.10
Detail of ink removal from the black paint area of Black on Maroon 1958
Tate
© Kate Rothko Prizel and Christopher Rothko, 2013
To increase the relatively slow evaporation rate of the benzyl alcohol and enhance the ink removal efficacy (by increasing polarity), while also minimising damage to the paint and glaze layers, a mixture of one part benzyl alcohol with one part ethyl lactate was also evaluated. This blend offered a lower contribution from dispersion forces (Table 4) and higher contributions from polar forces and hydrogen bonding, which contribute to the more efficient removal of the ink, while also potentially reducing the solvent effect on the underlying painting materials.23 This blend was eventually chosen as the most appropriate solvent for the removal of the ink from the black ‘figure’ area of the painting (fig.10). It offered a useful working time at the painting surface, was easy to control, and evaporated from the painting surface at an acceptable rate. However, it was found that the benzyl alcohol component of the system appeared to be causing the ink to travel deeper into the paint layer of the maroon field. Further tests showed that ethyl lactate on its own was a more efficient system for removing ink from this paint. After the majority of the ink had been cleared from the maroon ‘field’ using this system, the benzyl alcohol and ethyl lactate blend was then used to further reduce residues, although this was not always successful. Thus in order to minimse the impact of the solvents on the original paint, some ink residues remain within the maroon paint.
Conclusion
Testing solvent systems on the graffitied aged sections of the representative sample proved invaluable in establishing an effective system for removing the graffiti ink and recovering the painting surface. The behaviour of the samples, particularly in terms of their response to testing with a comprehensive range of solvents and application methods, enabled concise and minimal testing on the actual painting. Specifically, the sample facilitated an important practical understanding of two of the major risks associated with the ink removal process – pigment bleed and the potential for the ink to spread into unaffected areas – prior to the treatment of the painting itself. It also provided experience in how to prepare and handle the various sample materials used, and generated new knowledge on paint solubility, layer pick up, film hardness, gloss, wetting, toxicity and even the feasibility of some of the materials. The transfer of this knowledge to the treatment of Black on Maroon ensured that testing on the painting was minimised and carried out with precision. This was essential for enabling the exacting task of recovering Rothko’s delicately attenuated paint surface.
The treatment team was fortunate to have a body of technical knowledge and analysis on which to base this initiative, and the resources and pre-existing research collaborations to undertake extensive additional investigations. The extremely delicate process of removing a heavily coloured, alcohol-based, oligomeric ink from a degraded and complex painting surface necessitated a prolonged period of collaborative research prior to conservation treatment. This included examinations of the painting and of the graffiti ink, the design of solvent-based cleaning systems, the trialling of cleaning systems on a range of substrates, and the refinement of the final solvent application and softened ink removal processes. Furthermore, the creation of the representative sample facilitated periods of reflection involving Tate staff and external specialists, resulting in new insights and observations about Rothko’s complex painting, which will directly benefit the technical study and treatment of other works by Rothko in the collection. Most importantly, the research outcomes and carefully designed treatment strategy have allowed Black on Maroon to go back on display at Tate Modern alongside the other Seagram Murals.

