Conservators frequently report that the removal of surface dirt and grime from acrylic paintings can be problematic. While increasing amounts of research is being published on the analysis of acrylic paints1 and the historical use of acrylic paints by artists,2 relatively little has focused on the characterisation of the effects of surface cleaning treatments.3
One of the current areas of research at Tate is the evaluation of surface cleaning treatments for acrylic paintings, which includes the characterisation of the physical and optical changes to acrylic paint films after cleaning. This forms part of a broad research collaboration on modern paints involving many academic and cultural institutions, such as the National Gallery of Art where researchers are developing chromatographic and spectroscopic methods for the identification of additives in acrylic paints and the characterisation of extractable components. The Getty Conservation Institute (GCI) is continuing to develop techniques and methods for the quantitative identification of modern paints, as well as providing expertise and access to analytical equipment. Tate and GCI have also established a relationship with Exeter Advanced Technologies (X-aT), which has facilitated research into physical properties using thermal analytical techniques.4
Background
A recent review of the concerns surrounding the conservation of acrylic paintings has outlined the types of treatments currently in use by conservators and the typical problems encountered during and after the cleaning process.5 These concerns generally include: the inherent softness of acrylic paint layer(s); the imbibing of dirt; water and solvent sensitivity; and pigment removal. Many conservators of modern art have also mentioned an inherent concern for the extraction of low molecular weight species from the paint films, which were mostly unidentified but probably include surfactants and other additives.6 Most also commented upon the ease with which some form of surface disruption can be caused by the treatment process, leading to visual disturbances caused by overall changes in saturation, and/or more localised gloss or colour change. In the worst case, this can result in an unbalanced composition, or the loss of an original, or intended surface finish.
The response of acrylic paint films to surface cleaning can be further complicated by artists’ technique, and the presence of paint modifications including dilution and the addition of gloss or other paint mediums. The use of drying retardants, moisture, or high relative humidity atmospheres during the painting process can also affect film formation and influence the physical and optical properties of the paint. Equally importantly, the effects of natural ageing have not been the subject of prolonged research, particularly with respect to how ageing affects the response to cleaning.
The cleaning systems in general use have been adapted from methods established for oil paintings, and considerable expertise and knowledge has now built up through hands-on experience and information exchange. Most systems are currently based on aqueous and dry methods, with increasing application methods, and more complex systems containing various combinations of water, hydrocarbon solvents, surfactants, chelating agents and the blending of wet and dry systems.7
The principal aim of this research project is to assess the effects of cleaning via the characterisation of three fundamental properties – the physical, chemical and optical. Relevant physical properties include thermal and mechanical phenomena such as: glass transition temperature; the brittle-tough transition; flexibility; hardness; and the limits of stress fracture. Chemical investigations include changes in molecular weight distribution, changes in surface chemistry and the identification and possible quantification of additives and other products extracted from the paints. Optical properties include changes in colour and gloss, and an assessment of any physical disruption or ‘damage’ done to the surface during cleaning.
Method
Sample preparation

Fig.1
Adjustable film applicator and samples of titanium white paint on glass slides, primed cotton duck canvas and a Teflon® coated steel plate
© Tate Conservation Department, 2003

Table 1
Description of paints used for samples – preliminary experimentation
The following paints (tab.1) were drawn down onto three different substrates including: Teflon coated stainless steel sheets (Corus Firsteel Coated Strips, Walsall, UK); strips of a 10oz acrylic primed cotton duck canvas (Russell and Chapple, London); and cleaned glass slides (Agar Scientific, UK) to a dry thickness of approximately 80μm with a Sheen instruments adjustable film caster (fig.1) (Sheen Instruments, Kingston-upon-Thames, UK). At the time of experimentation, each sample had at least one months drying in ambient conditions, protected from dust, and it is noted therefore that the samples would probably not have been fully cured at this stage.8 A selection of the samples were thermally aged for a period of twenty four hours at 60°C in a Gallenkamp Vacuum oven.
Cleaning systems
A total of eighteen systems were selected (tab.2), aimed at representing most classes of the more commonly used cleaning systems that were mentioned in literature reviews and/or consultation with conservators of modern art. These include three dry cleaning methods, ten aqueous and four organic solvents. The organic solvents were included because they have occasionally been employed for overpaint/vandalism removal, and white spirits, or benzine, is being used more frequently by conservators concerned about the use of water on acrylic emulsion paintings.
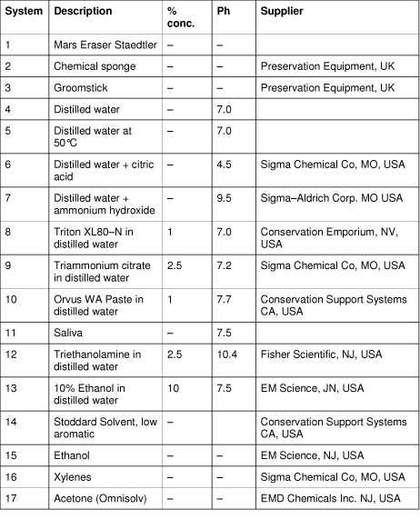
Table 2
Cleaning systems for preliminary experimentation
Application
The samples cleaned with liquid systems were immersed for either thirty minutes or one hour and removed with tweezers, or swab rolled for two minutes with individual surgically sterile prepared swabs (PURITAN, 100 x 6 inch (15cm) Cotton Tipped Applicators, Hardwood Products Company, Maine, USA). The swabs were loaded with liquid and then partially dried by rolling onto a paper towel. The swabs were reloaded after one minute or replaced if necessary. Some of the less volatile systems such as the Triton XL-80N, Orvus WA Paste and triammonium citrate, were cleared with a distilled water swab, or a five second water immersion for the free films. The dry systems were applied to the surface for one minute, then immediately brushed off with a light, dry, hog’s hair brush. After treatment, each sample had between one week and one months drying in ambient conditions before analysis. These initial application times were chosen to induce a degree of damage to the samples so that changes could be detected.
Instrumentation
Differential scanning calorimetry (DSC): At GCI, the method was based on ASTM E 1356,9 using a Mettler Toledo DSC822e instrument, operated with Stare software. Analysis was carried out under a nitrogen atmosphere with temperature range of -60°C to 100°C at a rate of 10°C/min. Three layers of paint were used and samples weighed prior to testing. At X-aT, the analysis was carried out on a Mettler 821e instrument, under a nitrogen atmosphere with a temperature range from -70 to 120°C at a rate of 10°C/minute. Two layers of paint were used and samples weighed prior to testing.
Dynamic mechanical analysis (DMA): Standard DMA analysis was carried out on a Polymer Labs MkIII DMTA in tensile mode. The temperature scan ranged from -50°C to 100°C at 3°C/min; the frequency was 1 Hz; strain at 2 (32μm approx peak to peak); the static load was a 0.1N reducing force; sample free length was 3mm; the clamp torque was set at 30cNm; with no averaging. Immersion DMA (creep) analysis was carried out on the Polymer Labs MkIII DMTA in tensile mode in ambient conditions. The frequency was 1 Hz; strain = 2 (32μm approx. peak to peak); a static load of 0.01N; sample free length of 3mm; clamp torque set at 30cNm; with no averaging.
Fourier transform infrared spectroscopy – attenuated total reflectance (FTIR-ATR): ATR spectra were obtained (200 scans at 4cm-1 resolution) on a Nic-Plan FTIR microscope (Nicolet) using a Spectra-Tech ATR objective with zinc selenide crystal. System was purged with dry air and data was processed with Omnic 4.1 software (Nicolet).
Atomic force microscopy (AFM): Non contact AFM Images were taken with an Explorer Scanning Microscope (Veeco Instruments, UK) using a nanosensor silicon nitride probe. Data was processed using SPMLabNT Ver 5.01 (Thermomicroscopes).
Environmental scanning electron microscopy (ESEM): Images were captured at 200x magnification with a Philips-FEI XL30 ESEM-FEG and adjusted using Photoshop 7.0. The accelerating voltage was set between 7 and 10 kV, the vacuum from 1 to 1.2 Torr, at a working distance of between 9.6 and 10.5mm.
Light microscopy: Images were captured with a WILD Heerbrugg stereomicroscope (Wild MB, Switzerland), illuminated from the right and top with swan neck lights (NCL 150, Volpi Manufacturing, NY, USA) using ACDSee 5.0 (ACS Systems Ltd) software and adjusted with Photoshop 7.0.
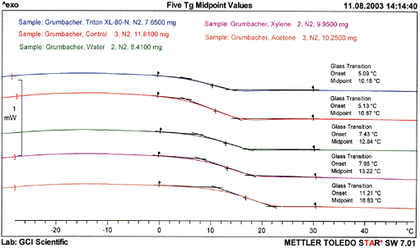
Fig.2
DSC curves – Tg region (second heating) of free films of Grumbacher titanium white paint samples, immersed in four cleaning systems and control
© The J. Paul Getty Trust. All rights reserved
Results
Physical properties
Differential scanning calorimetry (DSC)
DSC characterises the thermal transitions of a polymer taking place as a function of temperature. The temperature, direction i.e. exothermic or endothermic, and magnitude of thermal transitions are measured by cycling the sample through a heating and cooling program and comparing the amount of energy required to maintain its rate of temperature increase or decrease, with an inert reference pan under identical conditions. The difference between the heat required by the polymer and the heat required to heat the reference pan is expressed in the DSC curve as the heat absorbed and released from reversible and irreversible, physical or chemical transitions.
In this case, DSC was used to characterise the glass transition temperature (Tg) of free films of Grumbacher Titanium white acrylic emulsion paint after thirty minute immersions in the fourteen liquid cleaning systems. The Tg was taken at the mid point, and in order to remove any ‘history’ from the measurement that could modify the shape or size of the glass transition, such as mechanical stress or cooling effects, the Tg for the second heating was used for subsequent interpretations.
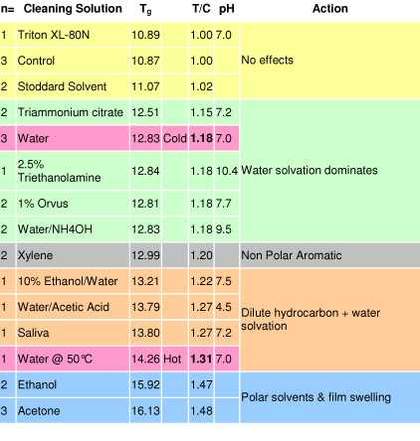
Table 3
DSC Tg results of Grumbacher titanium white paint after immersion in fourteen cleaning systems
The results (tab.3) confirm that a slight increase in Tg (equivalent to an increase in stiffness) was detectable after immersion in all cases except the Stoddard’s solvent and aqueous Triton XL-80N solution, both of which had little effect. The more polar organic solvents (ethanol and acetone) caused the largest increase in Tg, indicating that polarity may contribute to the extraction of plasticizing species within the paint film. The relevance of this upward shift in Tg on the long-term physical properties of the paint film is not yet clear, and will be the subject of further research.
In order to asses the effect of ageing on physical properties, two samples of the same Grumbacher titanium white paint film were analysed, including one that had been thermally aged for twenty hours at 60°C. The results (tab.4) show an upward shift in Tg for the thermally aged sample, confirming observations of increased film stiffness with age. However, as this paint may not have been fully cured, the shift may have also resulted from the further drying of the paint.
Dynamic mechanical analysis (DMA)
Standard DMA analysis
DMA is used to measure the mechanical and viscoelastic properties of materials as a function of temperature, time and frequency when subjected to a periodic stress. Viscoelastic materials such as polymers typically exist in two distinct states, they exhibit the properties of a glass (high modulus) at low temperatures and those of a rubber (low modulus) at higher temperatures. By scanning the temperature during a DMA experiment, changes of physical state can be observed and the glass transition temperature (Tg) can also be determined.
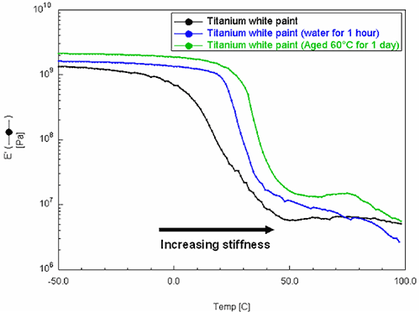
Fig.3
Overlayed DMA modulus curves of Grumbacher titanium white paint after immersion in water for 1 hour and thermal ageing for 24 hours at 60°C
© X-AT, University of Exeter
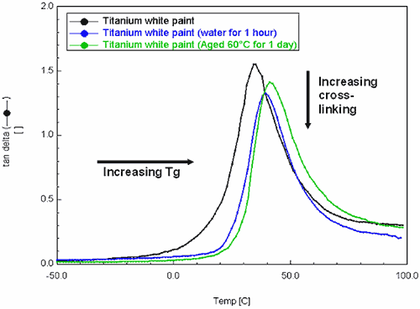
Fig.4
DMA overlaid tanδ curves of Grumbacher titanium white paint after immersion in water for 1 hour and thermal ageing for 24 hours at 60°C
© X-AT, University of Exeter
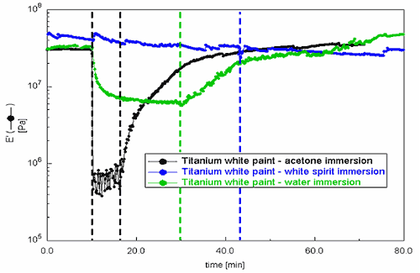
Fig.5
DMA immersion of Grumbacher titanium white free films – storage modulus (E') overlays
© X-AT, University of Exeter
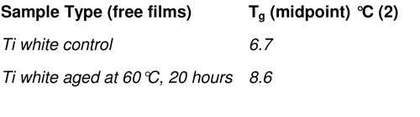
Table 4
DSC Tg results of Grumbacher titanium white paint – comparison between unaged and thermally aged paint for 20 hours at 60°C
Fig.3 shows the storage modulus (E’) curves overlaid for three samples of Grumbacher titanium white paint. The results, signified by an increase in temperature at the sharpest inflection point of each curve, indicate that both the water immersion and thermal ageing treatments resulted in an increase in the Tg of the paint. However, it is not possible to determine whether this increase is due to a loss of plasticising species or increased polymerization due to ageing.
Tan delta, or the ratio of the storage modulus over the loss modulus, (E′′/E′), is indicative of a material’s ability to dissipate energy. It can be used to measure Tg, and is taken as the temperature corresponding to the apex of the tan delta peak. It is noted that the Tg values produced by the DMA are higher than those produced by DSC – this is because the samples are frequency dependant, i.e., the higher the frequency used, the higher the Tg. When the results are viewed relatively, the DMA curves, (fig.4) show that both the water immersion and ageing treatments resulted in an increase in Tg.
Tan Delta also provides information on the cross-linking or polymer chain density of a paint film, providing another indicator of relative stiffness. The decreased apex heights of the immersed and aged samples indicate that the material may have cross-linked to a greater extent than the control, and/or that material has been extracted from the bulk film.
DMA immersion analysis: creep testing
DMA modulus data can also be acquired whilst the sample is fully immersed in a liquid. This provides information on the relative stiffness of the paints during immersion and the relative amount of creep each sample undergoes when subjected to a constant load. To assess this, free films of Grumbacher titanium white were immersed for varying periods in water, acetone and white spirits, and the storage modulus data collected over a period covering pre-immersion, immersion and post- immersion, as described in tab.5.

Table 5
DMA immersion regime for Grumbacher titanium white samples
Fig.5 displays the storage modulus data for each of the three samples. It is clear that the stiffness of the paint decreased significantly during the water and acetone immersions. However, the white spirits immersion appears to have had little effect on the stiffness of the paint. This is also reflected by the displacement curve (fig.6) which documents how much each sample stretches/creeps – via a combination of swelling and plasticising effects – during the immersion. Here the white spirits curve shows that this solvent facilitated relatively little displacement. It is also clear that water immersion resulted in significant displacement (up to 40%) and that acetone produced an extreme effect (over 120%). The displacement curves also shows that the acetone immersed sample did not return to its former dimensions after removal from the solvent, and that the water sample, although displaced by ten percent after drying, did recover to some extent.
Chemical properties
Fourier transform infrared spectroscopy – attenuated total reflectance (FTIR-ATR)
FTIR-ATR has proven to be a useful technique for the analysis of surfactants on the surface of acrylic media and paint films.10 Fig.7 contains three spectra from one sample of an unpigmented Golden acrylic paint medium painted onto glass. The top spectra is taken from the air-paint interface of the untreated film, the middle from the same air-paint interface after a one hour water immersion, and the lower spectra is taken from the back of the untreated film, i.e., at the substrate-paint interface.
The top most spectra, i.e., the film-air interface, contains four peaks at 2890, 1343, 1110, and 964cm-1 that correspond to the presence of a polyethylene oxide based surfactant, a common class of surfactant used in acrylic paint formulations.
The second and third spectra do not contain these peaks, thus confirming that the surfactant was removed by the one hour immersion, (second spectra) and that the surfactant content is drawn towards the air-film interface rather than the film-substrate interface, when painted onto a non-porous substrate (third spectra). These results have clear implications for water-based cleaning treatment; however, it is not yet clear whether the surfactant exudation is a continuous process and whether this has implications for the long-term stability of acrylic paints.
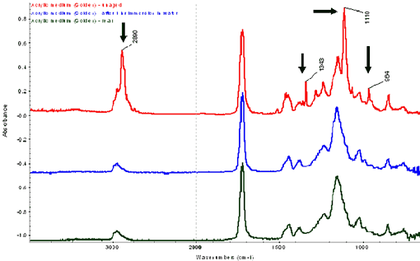
Fig.7
FTIR-ATR spectra of Golden acrylic medium – effect of drying and immersion
© Tate Conservation Department, 2003
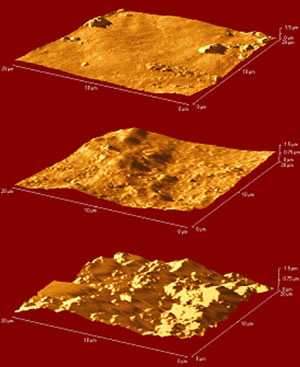
Fig.8
AFM images of Grumbacher titanium white paint: top: control; centre: water immersion; bottom: acetone immersion
© X-AT, University of Exeter
Optical properties
Atomic force microscopy (AFM)
Atomic Force Microscopy is an imaging technique used to extract visual, physical, thermal and electrostatic information from samples down to a possible resolution of nanometers. There are many different operating modes and resolutions that will be explored during the course of the research however, to date; AFM has been assessed for its ability to image the surface of acrylic paint films. Fig.8 shows AFM images of three pieces of Grumbacher titanium white free film. The uppermost sample is the control, the sample in the centre has been immersed in distilled water for a period of two hours, with a period of two hours drying in ambient conditions, and the lower sample has been immersed in acetone. The images confirm that AFM can successfully image the physical changes caused by these treatments and that the acetone immersion displays a considerably greater swelling and disruptive effect than water.

Fig.9
ESEM images of Golden titanium white on primed canvas, 2 min swab rolling. TL: control; TR: groomstick; BL: ethanol; BR: xylene, 200x
© The J. Paul Getty Trust. All rights reserved

Fig.10
ESEM images of Grumbacher gesso on glass slides, swab rolled with water for 2 mins, TL: control 200x; TR: control 500x; BL: water swabbed 200x; BR: water swabbed, 900x
© The J. Paul Getty Trust. All rights reserved
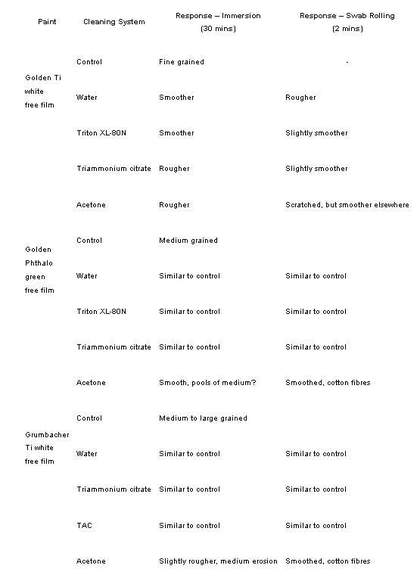
Table 6
Surface characteristics after cleaning drawn from photomicrographs of immersed and swab rolled free film samples of three diferent acrylic emulsion paints. Comparisons were made using the control sample from each paint type.
Environmental scanning electron microscopy (ESEM)
ESEM was chosen as the primary scanning electron imaging technique as it is known to cause less beam damage than high vacuum SEM, and negates the need to coat the samples. Separate experiments were carried out as follows:
- Grumbacher titanium white and Grumbacher acrylic gesso samples on glass, cleaned via swab rolling with distilled water.
- Golden titanium white on acrylic primed cotton duck canvas, cleaned with the seventeen systems listed in table 2.
- Comparison between free films of Golden titanium white, phthalocyanine green and Grumbacher titanium white each cleaned with distilled water, Triton XL-80N, acetone and triammonium citrate.
The ESEM images proved to be less informative than expected, and unsuccessful in discerning any reproducible differences in the samples exposed to water-based treatments.
However, the ethanol, xylene and acetone cleaned samples showed consistent evidence of damage in the form of deformation to the surface, visible medium erosion, the adhesion of cotton fibres and an undercutting of the surface layer, (fig.9). Some residues were also evident on the dry cleaned samples, even after clearing with a dry brush.
The Grumbacher acrylic gesso sample was also damaged by the water swabbing, in the form of dislodged chalk particles (fig.10), slight medium/surface erosion and collapsed areas.

Fig.11
Photomicrographs of Golden titanium white paint on primed canvas. TL: control, 25x; TR: control, 50x; CL: swabbed with distilled water, 25x CR: swabbed with distilled water, 50 x; BL: swabbed with xylene 25x; BR: swabbed with xylene, 50x, (scale bar = 1 mm)
© Tate Conservation Department, 2003
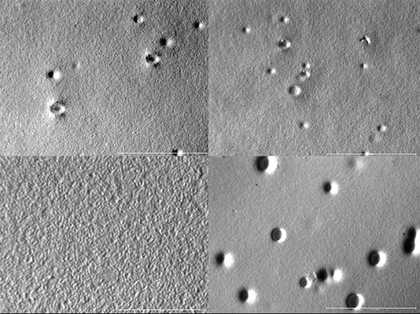
Fig.12
Comparison of immersed and swab rolled Golden titanium white paint samples at 50x: TL: control; TR: water immersed: CL: water swabbed; CR: triammonium citrate swabbed (scale bar = 1mm)
© Tate Conservation Department, 2003
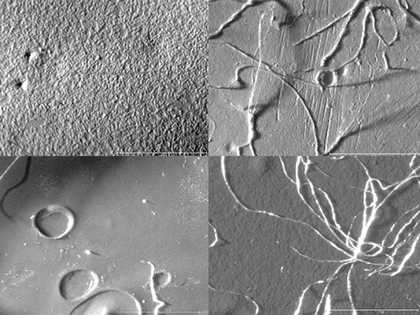
Fig.13
Comparison of Golden titanium white and phthalocyanine green samples. TL: titanium white, acetone immersed, 50x; TR: titanium white, acetone swabbed, 50x; BL: phthalocyanine green, acetone immersed, 25x; BR: phthalocyanine green, acetone immersed, 50x, (scale bar = 1mm)
© Tate Conservation Department, 2003
Light microscopy
In order to document the more macro features of acrylic paint surfaces, the samples studied with ESEM were also examined with a stereo microscope. The resulting micrographs (fig.11) confirmed that no obvious surface damage was caused by any of the water-based cleaning solutions, and that residues remained on the dry-cleaned samples. In addition, the damage caused by swab rolling with organic solvents was more visible under the stereomicroscope.
The three acrylic paints cleaned with four different solutions (ESEM experiment three) were also examined under the stereomicroscope. The results (tab.6, fig.12 and fig.13) revealed differences in the surfaces of the paints when using different application methods, for example, the immersions tended to produce rougher surfaces than the swab rolling.
In most cases, the cleaned samples appeared similar to the controls with the exception of the acetone treatments and the Golden titanium white paint. Acetone appeared to either erode or scratch the surface, with the exception of the immersed Golden phthalocyanine green, which was smoothed, producing pools of what may be additives or redistributed medium on the surface (fig.13). The Golden titanium white paint appeared to be more affected by the aqueous treatments than the other two paints, although the experiment needs to be repeated before any definite conclusions can be drawn.
Conclusion
The aim of this exploratory research was to assess a number of analytical and imaging techniques for their ability to provide information on the physical, chemical and optical changes to acrylic paint films resulting from cleaning treatments. Each of the techniques discussed has been successful in providing certain information on the behaviour of acrylic paints within the experimental conditions used, although clearly further experimentation is required.
The thermal analysis techniques, such as DSC and DMA, detected upwards shifts in the Tg of several paint films, indicating that acrylic paints become less flexible after treatment. They were also able to discern differences in Tg between the fourteen wet systems, where the white spirits and 1% solution of Triton XL-80N in distilled water had little effect, whilst the more polar and heated systems induced the greatest increases in Tg. DMA also provided an indication of the relative creep of the immersed paint films, where the low aromatic white spirits had the least effect of the three solvents tested.
FTIR-ATR analysis confirmed that polyethylene oxide-based surfactants contained in acrylic paints tend to migrate to the paint-air interface, and are removable from the surface by water immersion. The technique should be sensitive, therefore, to assessing the relative degree of removal with each of the cleaning systems.
Of the several imaging techniques evaluated, AFM produced successful three dimensional images of the surface of immersed acrylic paint films down to a micrometer scale, and the ESEM system was successful in imaging the surface damage caused by organic solvents, such as acetone and xylene, but was less successful for the water-based systems. The stereomicroscope also proved to be a useful technique for assessing the more macro features of acrylic paint films and any changes upon cleaning, particularly with the use of raking light.
Tailoring research to approximate actual conservation practice is also paramount, and both ongoing and future work will address issues related to application times and methods available, as well as increase the range of cleaning systems and introduce some complex and novel cleaning methods. The effect of repeated treatments is also important to this research, as is the inclusion of dirty samples.
